In the ongoing battle against antimicrobial resistance (AMR), a new review published in *Frontiers in Microbiology* offers a comprehensive look at the microbial drivers, One Health perspectives, and global strategies needed to combat this pressing threat. Led by Ayman Elbehiry of the Department of Public Health at Qassim University in Saudi Arabia, the research integrates clinical, environmental, and agricultural dimensions to highlight both challenges and opportunities in the fight against AMR.
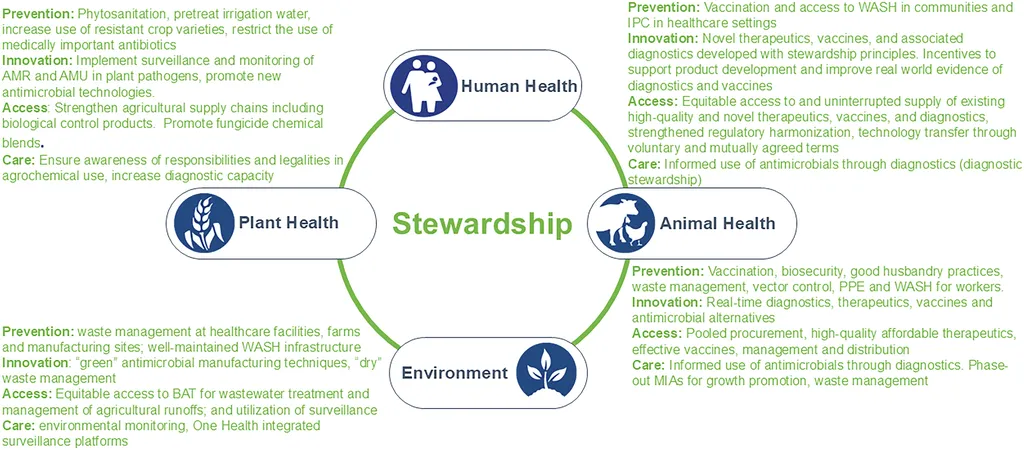
Antimicrobial resistance is a looming crisis, undermining modern medicine and threatening decades of progress in infection control. The review underscores the complex interplay of factors driving AMR, including microbial evolution, horizontal gene transfer, and the inappropriate use of antibiotics in human and veterinary medicine. Agricultural practices, environmental reservoirs, and uneven global regulation further complicate the issue. “The spread of resistance is not just a clinical problem—it’s an ecological and agricultural one,” Elbehiry explains. “Understanding these connections is crucial for developing effective solutions.”
One of the most significant findings is the role of soil, wastewater, and wildlife as conduits for spreading resistance elements. This highlights the need for a One Health approach, which recognizes the interconnectedness of human, animal, and environmental health. Advances in diagnostics, such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), whole-genome sequencing (WGS), digital PCR, and CRISPR-based assays, are transforming detection and surveillance. However, deployment remains uneven, particularly in low- and middle-income countries, where resources and infrastructure are often limited.
For the agriculture sector, the implications are substantial. The overuse of antibiotics in livestock and crop protection has long been a concern, contributing to the rise of resistant pathogens. The review emphasizes the need for antimicrobial stewardship that extends beyond hospitals, supported by decision support systems, artificial intelligence (AI), and community programs. “Agriculture must be part of the solution,” Elbehiry notes. “Sustainable practices and responsible antibiotic use in farming are essential to curb resistance and protect both human and animal health.”
The review also identifies persistent gaps in regulation, policy implementation, and incentives for antibiotic innovation. Priority directions include biomarker-guided prescribing, CRISPR-directed antimicrobials, microbiome-sparing therapeutics, and genomics-informed surveillance that integrates clinical and environmental data. Positioning the clinical microbiology laboratory as an operational hub could align rapid diagnostics, interpretive reporting, antimicrobial stewardship, and integrated surveillance on a common platform. This approach could translate laboratory results into actionable decisions, benefiting patient outcomes and public health.
As the world grapples with the consequences of antimicrobial resistance, this research offers a roadmap for future developments. By embracing a One Health framework and leveraging technological advancements, stakeholders in healthcare, agriculture, and policy can work together to mitigate the spread of resistance and safeguard the effectiveness of antimicrobial treatments. The review underscores the urgency of the situation and the need for coordinated global action to address this evolving threat.