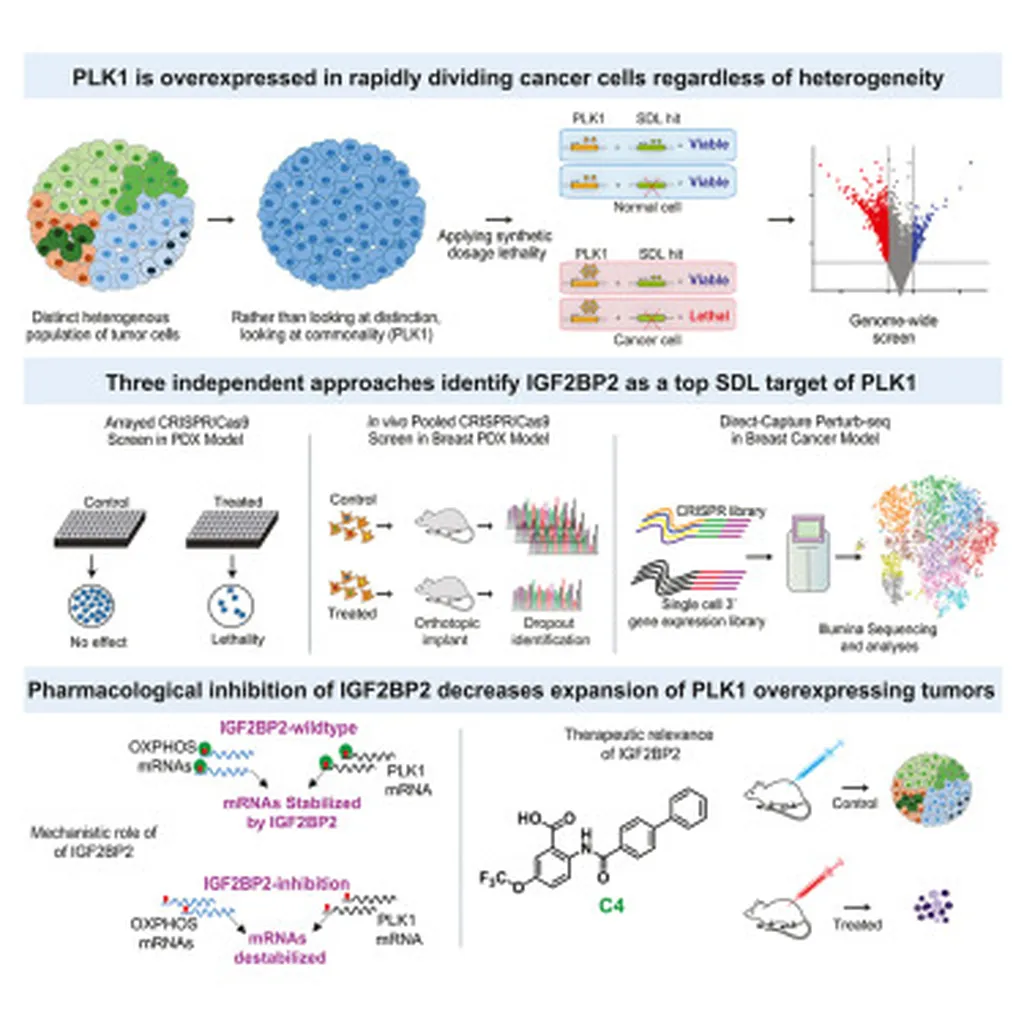
In the ever-evolving landscape of cancer research, scientists are continually seeking innovative strategies to combat the complexities of tumor heterogeneity and chromosomal instability (CIN). A recent study published in *Cell Genomics* has uncovered a promising new avenue for targeting cancers driven by Polo-like kinase 1 (PLK1) overexpression, offering hope for more effective therapies.
The research, led by Chelsea E. Cunningham from the Department of Oncology at the University of Saskatchewan, employed a genome-wide synthetic dosage lethality (SDL) screen to identify alternative therapeutic targets. This approach is particularly significant because direct inhibition of PLK1 has shown limited clinical benefits, despite its role in inducing CIN.
“Our goal was to find vulnerabilities in PLK1-overexpressing cancers that could be exploited for better therapeutic outcomes,” Cunningham explained. The team validated over 100 candidates using in vivo and in vitro secondary CRISPR screens, a testament to the thoroughness of their investigation.
One of the most striking findings was the identification of IGF2BP2 as a critical genetic dependency. When targeted, IGF2BP2 downregulation led to a significant reduction in PLK1 levels and restricted tumor growth. “This discovery opens up new possibilities for developing targeted therapies that can effectively tackle the heterogeneity and complexity of PLK1-driven malignancies,” Cunningham added.
The study also revealed that IGF2BP2 loss disrupted cellular energy metabolism and mitochondrial ATP production by downregulating PLK1 levels and genes associated with oxidative phosphorylation. This insight is crucial as it highlights the metabolic vulnerabilities of PLK1-overexpressing cancer cells, which are often addicted to higher metabolic rates.
The commercial implications of this research are substantial, particularly for the agriculture sector. Understanding the metabolic dependencies of cancer cells can lead to the development of novel agricultural biotechnologies aimed at improving crop resilience and yield. For instance, targeting similar metabolic pathways in plants could enhance their ability to withstand environmental stresses, ultimately benefiting farmers and the agricultural industry as a whole.
Moreover, the use of direct-capture Perturb-seq to assess the transcriptional consequences and viability of each SDL perturbation at a single-cell resolution represents a significant advancement in the field. This technique provides a detailed map of how genetic perturbations affect cellular behavior, paving the way for more precise and personalized cancer treatments.
As we look to the future, this research has the potential to shape the development of new therapeutic strategies and agricultural technologies. By leveraging the insights gained from this study, scientists can continue to push the boundaries of what is possible in both cancer treatment and agricultural innovation.
In summary, the work of Chelsea E. Cunningham and her team offers a novel therapeutic strategy against PLK1-driven heterogeneous malignancies, with far-reaching implications for both the medical and agricultural sectors. As the field continues to evolve, the integration of advanced technologies and interdisciplinary approaches will be key to unlocking new possibilities for improving human health and agricultural productivity.