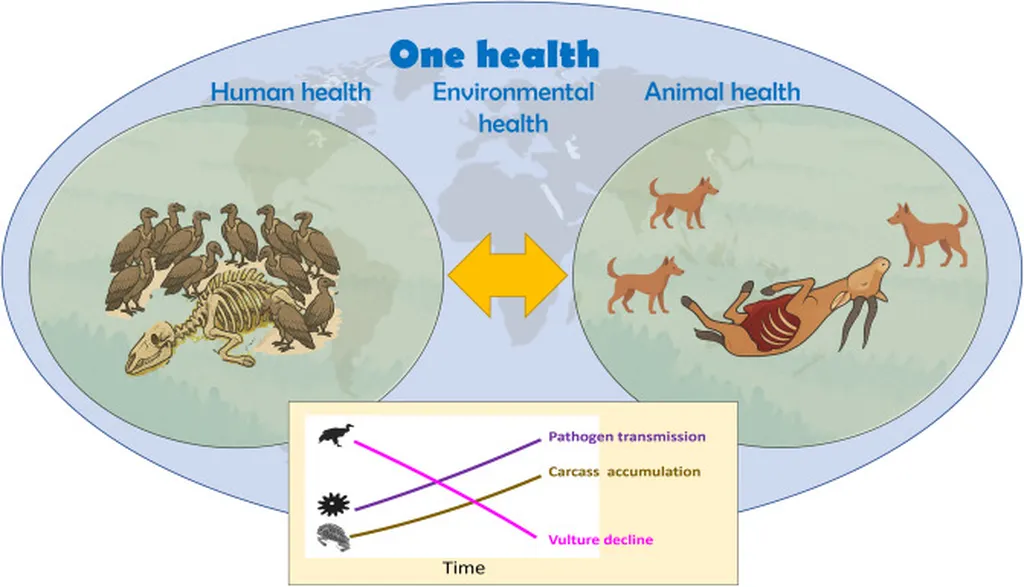
In the vast, interconnected web of life, the role of vertebrate carcasses in disease transmission has long been overlooked, but new research is shedding light on their critical importance. A recent study published in *Frontiers in Veterinary Science* highlights how carcasses serve as both a vital resource and a potential hotspot for pathogen transmission, with significant implications for agriculture and public health.
The research, led by Katie A. Barton from the Sydney School of Veterinary Science at the University of Sydney, underscores the growing frequency of mass mortality events (MMEs) in wildlife, driven by factors like climate change, agricultural intensification, and urbanization. These events, coupled with declining populations of scavengers like vultures, are altering disease dynamics in ways that could have far-reaching consequences for livestock and human health.
“Carcasses are not just passive entities; they are active participants in the transmission of pathogens,” Barton explains. “They can facilitate cross-species and trophic spillover events, making them a critical component of disease surveillance.”
The study highlights how scavengers play a dual role in this process. On one hand, they can remove infectious material from the environment by consuming carcasses. On the other, their mobility and social behavior can disperse pathogens over large areas, potentially amplifying disease transmission.
Advances in technology are now enabling researchers to track these interactions with unprecedented detail. Remote sensing, camera traps, GPS telemetry, and machine learning are being used to identify transmission hotspots, while metagenomic sequencing allows for the detection of known and novel pathogens in the necrobiome—the microbial communities associated with carcasses. Portable sequencing platforms are even making it possible to conduct field-based surveillance, bringing the lab to the field.
For the agriculture sector, the implications are significant. Livestock are not immune to the dynamics at play in these ecosystems. As Barton notes, “Understanding these interactions can help us predict and mitigate disease outbreaks, protecting both wildlife and domestic animals.”
The integration of carcass-based surveillance into One Health frameworks—an approach that recognizes the interconnection between human, animal, and environmental health—could revolutionize disease monitoring and preparedness. By fostering collaboration among ecologists, epidemiologists, and data scientists, this approach offers a proactive strategy for early outbreak detection and ecosystem health monitoring.
As climate-driven mortality events are projected to increase, incorporating carcass-scavenger networks into disease surveillance strategies could prove invaluable. It’s a complementary approach that enhances our ability to monitor and mitigate emerging infectious diseases, ultimately safeguarding agricultural productivity and public health.
In a world where disease emergence is accelerating, this research offers a timely reminder of the interconnectedness of all living things. By embracing interdisciplinary collaboration and leveraging cutting-edge technology, we can better navigate the complexities of disease transmission and build a healthier, more resilient future for all.
The study, led by Katie A. Barton from the Sydney School of Veterinary Science at the University of Sydney, was published in *Frontiers in Veterinary Science*.