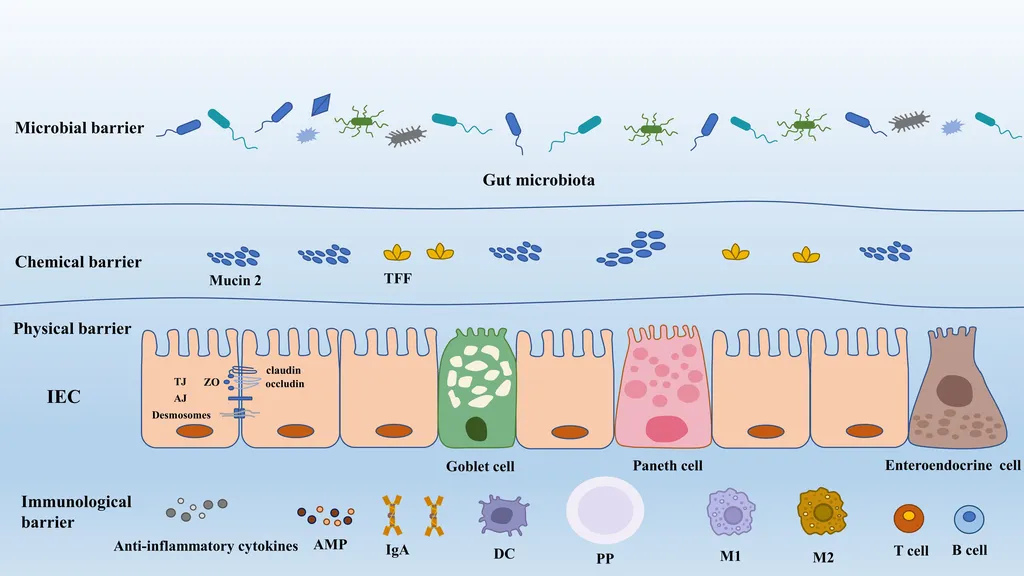
In the intricate ecosystem of the gut, a delicate balance between microbiota, epithelial barriers, and host immunity is crucial for maintaining intestinal health. Disruptions to this triad can spark inflammatory cascades, leading to various gastrointestinal disorders. Recent research published in the journal *Frontiers in Microbiology* (translated from Chinese as “Microbiology Frontiers”) sheds light on how two compounds—ferulic acid and its derivative N-Feruloylserotonin—can mitigate lipopolysaccharide (LPS)-induced acute inflammation, offering promising avenues for intestinal health preservation and inflammatory disease prevention.
Led by Xiangdong Hu from the Department of Gastroenterology at The First People’s Hospital of Wenling in China, the study explores the multifaceted effects of these compounds on gut microbiota, serum metabolome, and transcriptional networks. The findings reveal that both compounds not only attenuate LPS-induced intestinal pathology in murine models but also suppress pro-inflammatory cytokine expression. This dual action underscores their potential as therapeutic agents in managing intestinal inflammation.
One of the most compelling aspects of the research is its integrated approach, which examines the gut microbiome, serum metabolome, and transcriptional networks simultaneously. “Our study provides a comprehensive view of how ferulic acid and N-Feruloylserotonin modulate the gut environment to combat inflammation,” says Hu. This holistic perspective is crucial for understanding the complex interplay between dietary compounds and gut health.
The research identified several beneficial metabolites, including 1-naphthalenesulfonic acid, which were enriched by the administration of these compounds. Additionally, probiotic taxa such as Ruminococcaceae, Muribaculaceae, Lachnospiraceae, Bifidobacteriaceae, Prevotellaceae, Roseburia, Blautia, and Butyricicoccus were found to flourish, while pathobionts like Proteobacteria, Gammaproteobacteria, Enterobacterales, and Bacillus were suppressed. This shift in microbial composition is a key indicator of improved gut health and reduced inflammation.
Transcriptomic profiling further revealed that the compounds modulate several critical signaling pathways, including antigen processing and presentation, NF-κB, MAPK, and PI3K-Akt. Key regulatory targets identified include Pik3cd, H2-DMb1, H2-Oa, Kdr, Fgfr3, Il1r2, Rac, Irak4, Traf6, Ticam1, Rip1, and Rip3. These findings provide a mechanistic foundation for understanding how ferulic acid and N-Feruloylserotonin exert their anti-inflammatory effects.
The implications of this research extend beyond the realm of gastrointestinal health. As our understanding of the gut microbiome’s role in overall health continues to grow, the potential applications of these compounds in other areas, such as metabolic and immune disorders, are becoming increasingly apparent. “This work not only establishes a mechanistic foundation for deploying ferulic acid and N-Feruloylserotonin in intestinal health preservation but also provides novel insights into microbiota-homeostasis crosstalk,” Hu notes.
For the energy sector, the research highlights the potential for developing novel bio-based products that can enhance gut health and, by extension, overall well-being. As the demand for sustainable and health-promoting products grows, the integration of these compounds into dietary supplements, functional foods, and even pharmaceuticals could open new markets and opportunities.
In conclusion, the study by Hu and colleagues represents a significant step forward in our understanding of how dietary compounds can modulate gut health and inflammation. By elucidating the complex interactions between gut microbiota, metabolome, and transcriptome, this research paves the way for innovative therapeutic strategies that could benefit a wide range of individuals. As the field continues to evolve, the insights gained from this study will undoubtedly shape future developments in gut health and beyond.