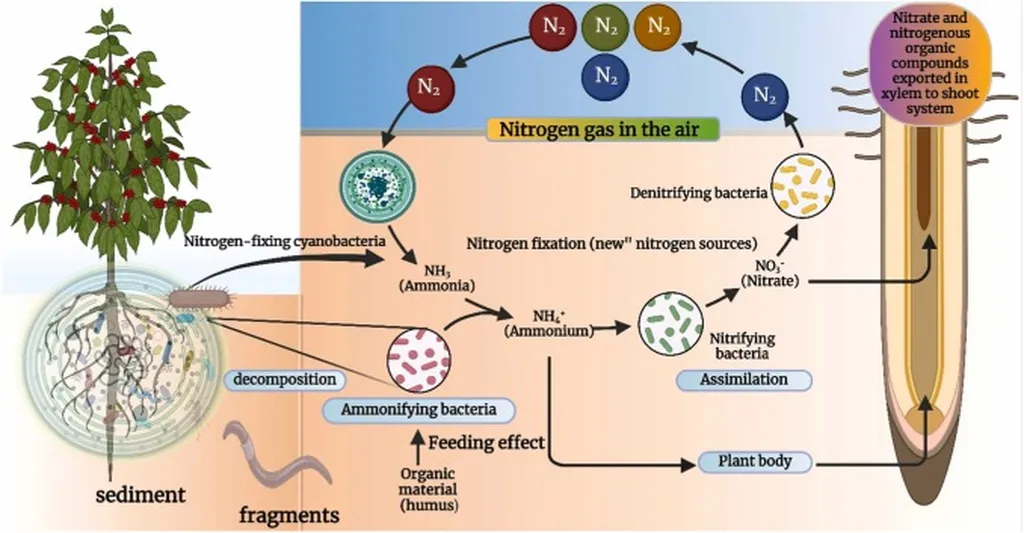
In the quest for sustainable agriculture and renewable energy solutions, scientists are turning to an unlikely ally: cyanobacteria. These ancient microorganisms, known for their role in oxygen production, are now being studied for their potential to revolutionize the way we fertilize crops and produce biofuels. A recent study led by James Young from the Department of Biology and Microbiology at South Dakota State University has uncovered a novel approach to identifying nitrogen-fixing cyanobacteria, a discovery that could have significant implications for the agricultural and energy sectors.
Nitrogen fixation, the process by which certain bacteria convert atmospheric nitrogen into a form that plants can use, is a highly sought-after trait in agriculture. Cyanobacteria that possess this ability, known as diazotrophic cyanobacteria, can potentially reduce the need for synthetic fertilizers, which are energy-intensive to produce and contribute to environmental pollution. “Identifying new diazotrophic strains is crucial for advancing agricultural biotechnology,” Young explains. “Our goal is to find strains that can enhance crop productivity while minimizing environmental impact.”
The study, published in the journal Metabolites (translated to English as “Metabolites”), employed a cheminformatic approach to predict diazotrophic cyanobacteria based on their secondary metabolites. Secondary metabolites are compounds produced by organisms that are not essential for their growth and reproduction but often play a role in their interaction with the environment. By analyzing the chemical structure similarity of these metabolites, the researchers developed a predictive model that achieved an impressive 88% accuracy rate.
The model was trained on a dataset of 133 manually labeled metabolites and then applied to a larger set of 1980 unlabeled metabolites. This approach allowed the researchers to prioritize likely diazotrophic strains among the unlabeled data, providing a ranked list of potential candidates for further study. “This method allows us to narrow down the vast diversity of cyanobacteria to a manageable number of promising strains,” Young notes.
One of the intriguing findings of the study was that diazotrophic-associated metabolites showed similar toxicity levels to non-diazotrophic metabolites in rats but were less toxic to Daphnia magna, a small water flea. This suggests that these metabolites are not primarily serving a defensive role against predators. Instead, many of these metabolites were cyclic peptides, which could potentially act as signaling molecules. The high nitrogen content in these metabolites further supports their role in nitrogen fixation.
The implications of this research extend beyond agriculture. In the energy sector, diazotrophic cyanobacteria could be harnessed for biofuel production. These microorganisms can convert solar energy into chemical energy through photosynthesis, making them a promising source of renewable energy. By identifying new diazotrophic strains, researchers can potentially enhance the efficiency of biofuel production processes.
The study’s findings open up new avenues for exploring the potential of cyanobacteria in sustainable agriculture and energy production. As Young puts it, “This research is just the beginning. There’s a wealth of untapped potential in these microorganisms, and we’re excited to see where this leads us.”
In the coming years, we can expect to see further advancements in the field of agricultural biotechnology, driven by innovative approaches like the one described in this study. By leveraging the power of cheminformatics and predictive modeling, researchers are paving the way for a more sustainable and efficient future. The discovery of new diazotrophic cyanobacteria strains could not only enhance crop productivity but also contribute to the development of cleaner energy solutions, ultimately benefiting both the environment and the economy.