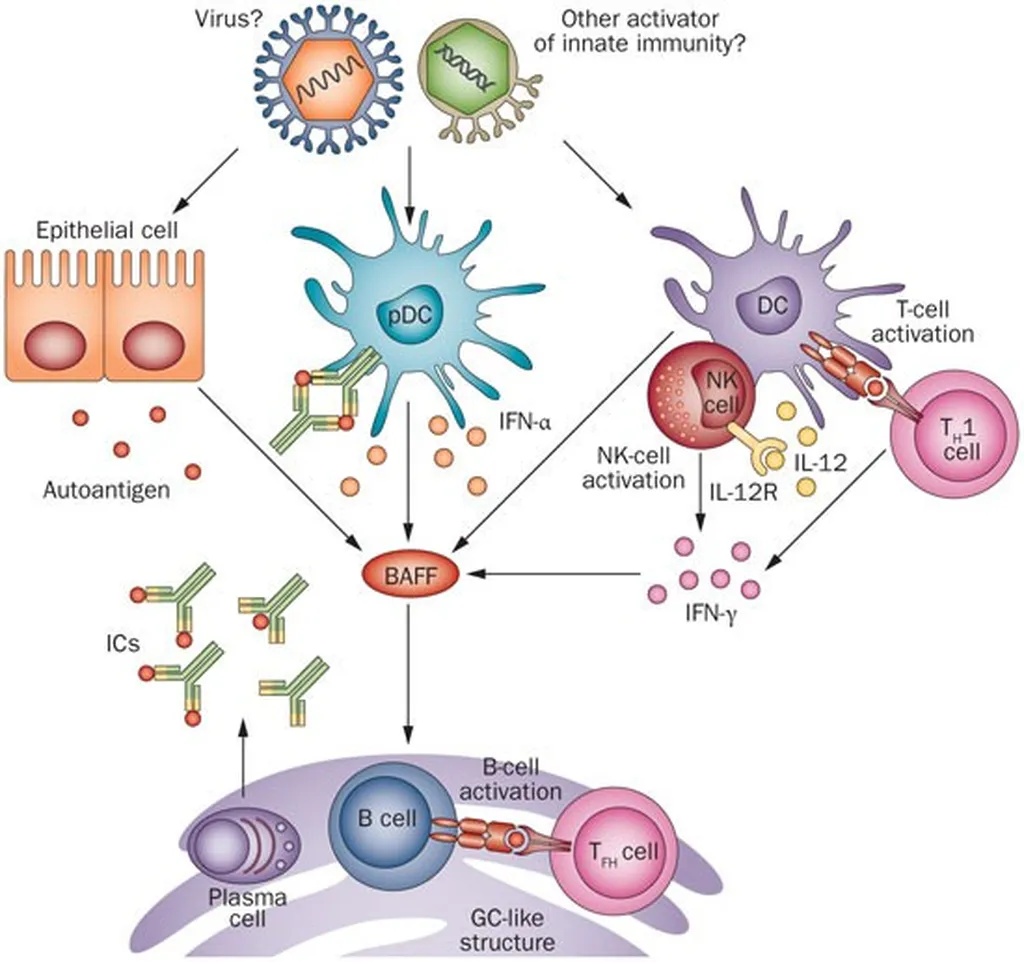
In a groundbreaking study published in the journal *Nature Communications* (translated as “Nature Communications”), researchers have unveiled new insights into the molecular mechanisms of Sjögren’s disease (SjD), an autoimmune condition that affects the salivary glands. The study, led by Jun Inamo from the Division of Rheumatology at Keio University School of Medicine, employed advanced single-cell technologies to dissect the cellular and transcriptional signatures associated with different autoantibody profiles in SjD patients.
Sjögren’s disease is characterized by immune-mediated destruction of salivary glands, leading to dry mouth and other symptoms. While autoantibodies like anti-SSA and anti-centromere (CENT) are known to correlate with distinct clinical manifestations, the underlying molecular features have remained elusive until now. Inamo and his team utilized single-cell RNA sequencing, T cell and B cell receptor sequencing, and spatial transcriptomics to explore the salivary gland lesions of SjD patients.
The findings revealed that GZMB + GNLY + CD8+ T cells are a prominent subset across different autoantibody statuses, underscoring their pivotal role in the disease’s pathogenesis. “This subset of T cells is highly expanded in SjD patients, regardless of their autoantibody profile,” Inamo noted. “This suggests a common pathway in the immune response that could be targeted for therapeutic intervention.”
However, the study also highlighted differences based on autoantibody profiles. Memory B cells were found to be more abundant in anti-CENT-positive patients, while cytokine signaling varied significantly. Anti-SSA-positive patients exhibited an activated interferon signature, whereas TGFβ signaling was enhanced in anti-CENT-positive patients. “These differences in cytokine signaling could explain the varied clinical presentations seen in SjD patients,” Inamo explained.
One of the most intriguing findings was the identification of THY1 + fibroblasts as key players in orchestrating inflammation within the salivary glands. These fibroblasts express complement genes and chemokines, suggesting they act as hubs for immune cell recruitment and activation. “Understanding the role of these fibroblasts could open new avenues for targeted therapies,” Inamo said.
The implications of this research are far-reaching. By elucidating the molecular mechanisms underlying SjD, the study paves the way for the development of personalized therapeutic strategies. “This research not only deepens our understanding of SjD but also provides a framework for exploring other autoimmune diseases with similar mechanisms,” Inamo added.
As the field of autoimmune research continues to evolve, this study serves as a testament to the power of advanced single-cell technologies in unraveling complex disease mechanisms. The findings could inform the development of targeted therapies, ultimately improving the lives of SjD patients and those affected by similar conditions. With the publication of this study in *Nature Communications*, the scientific community is one step closer to unlocking the secrets of autoimmune diseases and developing more effective treatments.