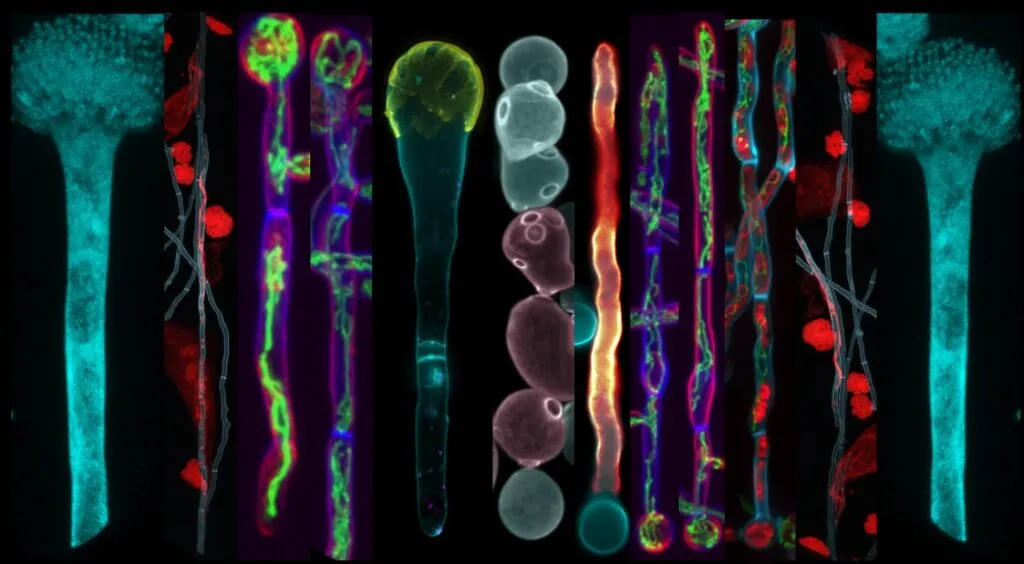
In the intricate dance of plant-pathogen interactions, a recent study has shed light on a crucial step that could reshape our understanding of how fungal pathogens invade and manipulate their hosts. The research, led by Jiwon Choi from the Department of Agricultural Biotechnology at Seoul National University, focuses on the nuclear localization sequence (NLS) of MoHTR2, a nuclear effector of the rice blast fungus Magnaporthe oryzae. Published in The Plant Pathology Journal, the study delves into the molecular mechanisms that enable fungal pathogens to hijack host cells, with potential implications for developing more resilient crops and innovative agricultural technologies.
Magnaporthe oryzae, a notorious plant fungal pathogen, secretes nuclear effectors that reprogram the transcription of host immunity-associated genes. These effectors, including MoHTR2, are known to enter the host nucleus, but the specific sequences and mechanisms facilitating this entry have remained largely mysterious. Choi’s research has identified a critical motif, 53HH54, within MoHTR2 that is essential for its nuclear localization. This discovery challenges the conventional understanding that all nuclear effectors rely on the classical importin α-mediated pathway.
“Our findings reveal that MoHTR2 does not interact with rice importin αs or βs, indicating a non-classical pathway for its nuclear entry,” Choi explains. This insight not only broadens our knowledge of fungal pathogenesis but also opens new avenues for targeting these pathways to disrupt the infection process. By performing serial truncation and site-directed mutagenesis, the research team pinpointed the double histidine motif as a key player in the nuclear localization of MoHTR2. Furthermore, the study found that deleting this core NLS significantly reduced the invasive hyphal growth and lesion formation by M. oryzae, underscoring its role in pathogenicity.
The implications of this research extend beyond academic curiosity. Understanding the molecular intricacies of nuclear effectors can lead to the development of novel fungicides and genetically engineered crops with enhanced resistance to fungal pathogens. For the agricultural sector, this means the potential for higher crop yields and reduced losses due to fungal infections, which are a significant economic burden globally. Additionally, the insights gained from this study could inspire innovations in biotechnology, such as the creation of synthetic effectors for targeted gene manipulation in plants.
As we continue to unravel the complexities of host-pathogen interactions, studies like Choi’s pave the way for groundbreaking advancements in agriculture and biotechnology. By elucidating the mechanisms behind the nuclear localization of fungal effectors, we move closer to developing sustainable solutions for crop protection and improving global food security. The journey to decipher these molecular interactions is far from over, but each discovery brings us one step closer to a future where agriculture is more resilient and productive.