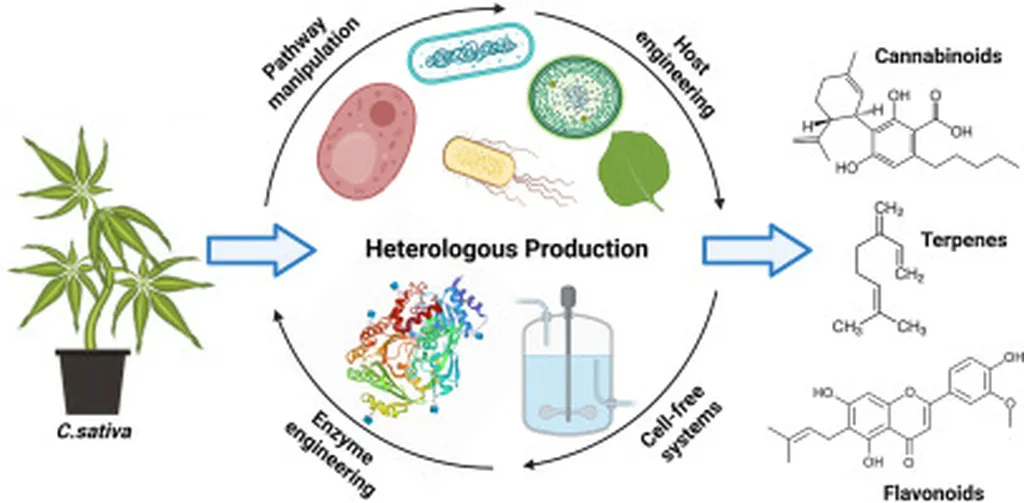
In a groundbreaking development that could reshape the agricultural and pharmaceutical landscapes, researchers have successfully engineered fungi to produce cannabinoids, offering a sustainable and scalable alternative to traditional cannabis cultivation. This innovation, detailed in a recent mini-review published in *Frontiers in Fungal Biology*, opens new avenues for the production of therapeutic compounds like Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which have shown promise in treating pain, inflammation, and neurological disorders.
The study, led by Madira Coutlyne Manganyi from the Department of Biological and Environmental Sciences at Sefako Makgatho Health Sciences University in Pretoria, South Africa, highlights the potential of synthetic biology and metabolic engineering to overcome the limitations of traditional cannabis farming. “Engineered fungi provide a flexible and controllable platform for cannabinoid production, addressing issues like crop yield variability, regulatory constraints, and environmental impact,” Manganyi explained.
Cannabinoids have long been prized for their therapeutic benefits, but their production has been hindered by the challenges of cultivating cannabis plants. Factors such as climate conditions, regulatory hurdles, and the plant’s slow growth cycle have limited supply. The emergence of microbial biosynthesis, particularly in fungi, offers a solution that could revolutionize the industry. Fungi are known for their metabolic versatility and ease of genetic manipulation, making them ideal candidates for producing complex secondary metabolites like cannabinoids.
The research outlines several key strategies that have enabled this breakthrough. Pathway reconstruction and enzyme optimization have been crucial in ensuring that fungi can efficiently synthesize cannabinoids. Additionally, the use of CRISPR-Cas9 genome editing has allowed for precise modifications, enhancing the fungi’s ability to produce these valuable compounds. “By leveraging these advanced biotechnological tools, we can optimize fungal strains to maximize cannabinoid yield and minimize production costs,” Manganyi noted.
Despite these advancements, challenges remain. Product toxicity, metabolic burden, and regulatory considerations are among the hurdles that researchers must address. However, the potential benefits are substantial. The ability to produce cannabinoids in engineered fungi could lead to a more consistent and cost-effective supply chain, benefiting the pharmaceutical, nutraceutical, and industrial sectors.
Looking ahead, the field of systems biology and bioprocess optimization holds promise for further advancements. The production of rare cannabinoids, which are difficult to obtain from traditional sources, could become more feasible. This could open up new therapeutic possibilities and expand the market for cannabinoid-based products.
The implications of this research extend beyond the laboratory. For the agriculture sector, the shift towards microbial biosynthesis could reduce the need for large-scale cannabis cultivation, alleviating pressure on land and resources. It could also provide new opportunities for farmers to diversify their operations by integrating biotechnological production methods.
As the world continues to explore the therapeutic potential of cannabinoids, the development of engineered fungi represents a significant step forward. This innovation not only addresses current production challenges but also paves the way for future advancements in microbial biotechnology. With continued research and collaboration, the agricultural and pharmaceutical industries could see a transformative shift towards more sustainable and efficient production methods.