In the intricate world of microbial interactions, a recent study published in the journal ‘Biofilm’ has shed new light on the complex dynamics between Staphylococcus aureus and Candida albicans, two pathogens often found together in clinical infections. The research, led by Yaqin Li from the Department of Laboratory Medicine at The Second Affiliated Hospital of Shantou University Medical College and the School of Food Science and Engineering at South China University of Technology, delves into the nuanced relationship between these two species, offering insights that could reshape our understanding of polymicrobial infections and their treatment.
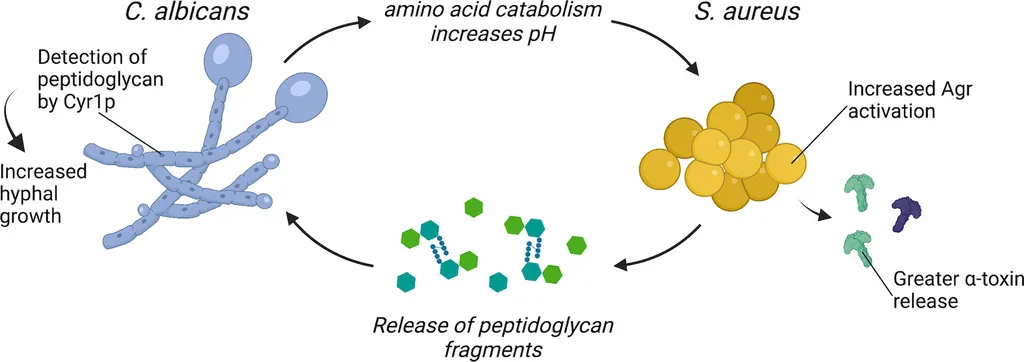
The study reveals a fascinating interplay between S. aureus and C. albicans, where the environment plays a pivotal role in determining the dominant species. In planktonic, or free-floating, conditions, C. albicans takes the upper hand, suppressing the growth of S. aureus. However, when these microbes form biofilms—complex communities that adhere to surfaces—the scenario changes dramatically. “In biofilm conditions, we observed a mutual adaptation,” Li explains. “The hyphae-competent C. albicans forms dual-species biofilms with S. aureus, leading to substantial biomass accumulation and enhanced biofilm cohesion and resistance.”
This mutual adaptation is not just a passive coexistence but a dynamic interaction that influences the structure and resilience of the biofilm. The study found that the colonization order of the species affects the biofilm architecture, with highly mature biofilms exhibiting dense network structures and increased C. albicans hyphal formation. The mechanical measurements revealed an elastic modulus of up to 10 Pa, indicating enhanced biofilm rigidity and structural integrity.
One of the most intriguing findings is the stage-dependent role of C. albicans hyphae. During the proliferating phase, the hyphal form of C. albicans facilitates the proliferation of S. aureus, highlighting the intricate and temporally dynamic nature of their interaction. “The hyphal contribution of C. albicans is crucial during the proliferating phase,” Li notes. “This stage-dependent interaction underscores the complexity of polymicrobial infections and the need for targeted interventions.”
The implications of this research extend beyond clinical settings into the agricultural sector. Polymicrobial biofilms are a significant concern in food processing and storage, where they can lead to spoilage and contamination. Understanding the dynamics between S. aureus and C. albicans could lead to the development of more effective strategies for preventing and treating biofilm-associated infections in agricultural environments. For instance, targeted interventions that disrupt the mutual adaptation between these species could enhance the safety and shelf life of food products.
Moreover, the study’s findings could pave the way for innovative agricultural technologies aimed at controlling microbial biofilms in food processing facilities. By leveraging the insights into the stage-dependent interactions between S. aureus and C. albicans, researchers and developers can create more effective cleaning and disinfection protocols, as well as bio-based treatments that specifically target these biofilms.
In conclusion, the research led by Yaqin Li offers a comprehensive look at the complex interactions between S. aureus and C. albicans, highlighting the importance of understanding polymicrobial dynamics in both clinical and agricultural contexts. As we continue to unravel the intricacies of microbial interactions, the potential for developing targeted and effective interventions grows, promising a future where biofilm-associated infections are better managed and controlled.