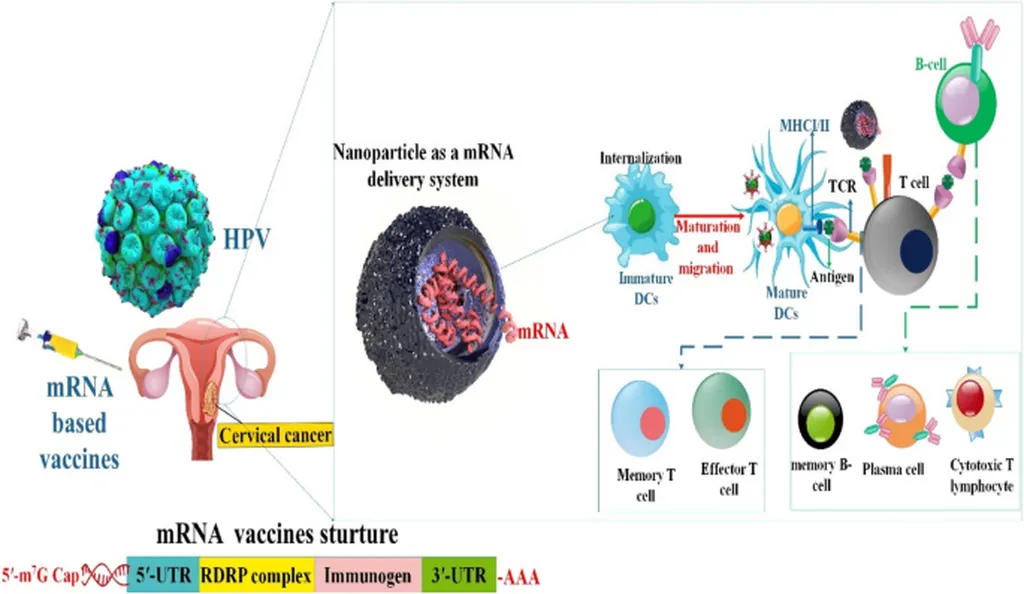
In a groundbreaking development that could revolutionize cervical cancer prevention and therapy, researchers have designed a multi-epitope vaccine targeting high-risk human papillomavirus (HPV) types 16, 18, 33, and 45. This innovative approach, detailed in a study published in *Bioinformatics and Biology Insights*, leverages immunoinformatics to create a vaccine with both prophylactic and therapeutic potential, addressing a significant gap in current medical interventions.
The study, led by Md. Touki Tahamid Tusar from the Department of Microbiology and Hygiene at Bangladesh Agricultural University, focuses on the L1 and E7 proteins of HPV, which are crucial for viral replication and oncogenesis. Existing vaccines primarily target the L1 protein for prevention but lack therapeutic efficacy. By incorporating epitopes from both L1 and E7 proteins, the researchers aim to enhance both preventive and therapeutic outcomes.
“Our goal was to design a vaccine that not only prevents HPV infection but also has the potential to treat existing infections and associated cancers,” said Tusar. The vaccine design process involved predicting epitopes using the Immune Epitope Database (IEDB) and ABCpred, followed by rigorous screening for antigenicity, immunogenicity, safety, conservancy, population coverage, and homology. The selected epitopes were then assembled into a vaccine construct using suitable linkers and a 50-S L7/L12 adjuvant.
The modeled vaccine demonstrated robust interactions with toll-like receptors TLR2 and TLR4, indicating strong immune system engagement. Molecular dynamics simulations validated the structural stability of the vaccine, and molecular mechanics with generalized born and surface area solvation (MM/GBSA) analysis showed favorable binding free energies. Immune simulations using C-IMMSIM revealed that the vaccine could elicit strong and long-lasting cellular and humoral immune responses, as well as significant cytokine production.
One of the most promising aspects of this research is its potential impact on the agriculture sector. While the immediate application is in human health, the techniques and insights gained from this study could be adapted for veterinary vaccines. Livestock, particularly in developing countries, are often affected by various strains of HPV and other viral infections. A similar multi-epitope vaccine approach could be developed to protect agricultural animals, improving their health and productivity.
“Our findings suggest that this vaccine could be a game-changer in the fight against HPV-related diseases,” said Tusar. “The next steps involve laboratory and animal studies to validate its efficacy and safety. If successful, this could pave the way for a new era in both human and animal health.”
The study also highlights the importance of codon optimization for efficient expression in host systems. The researchers optimized the vaccine construct for Escherichia coli K12, achieving a guanine-cytosine content of 50.69% and a Codon Adaptation Index of 0.97. In silico cloning into the pET28a(+) vector confirmed high expression potential, further supporting the feasibility of large-scale production.
This research not only advances our understanding of HPV and cervical cancer but also opens new avenues for developing multi-epitope vaccines against other infectious diseases. The integration of immunoinformatics and advanced computational tools has enabled the design of a vaccine with broad implications for both human and animal health. As the study progresses to clinical trials, the agricultural sector stands to benefit from the adaptation of these technologies, potentially leading to improved livestock health and productivity.
The study was published in *Bioinformatics and Biology Insights* and led by Md. Touki Tahamid Tusar from the Department of Microbiology and Hygiene at Bangladesh Agricultural University.