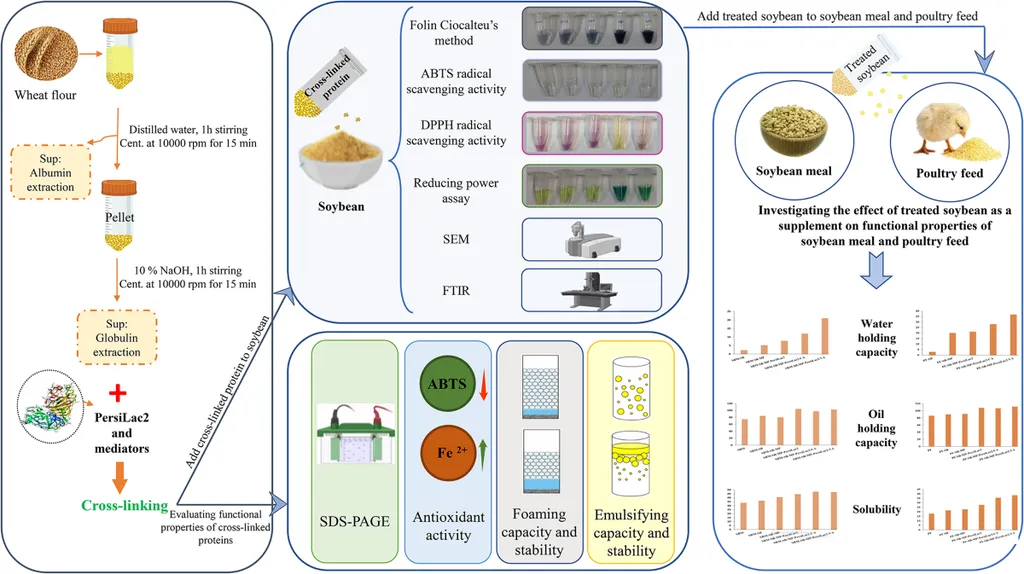
In a groundbreaking study published in *BMC Biotechnology*, researchers have unveiled a novel approach to enhance the nutritional value and functionality of soybean meal (SBM), a staple protein source in both human and animal diets. The research, led by Tina Rouzban from the Department of Cell and Molecular Biology at Kharazmi University, explores the use of metagenomic laccase-catalyzed crosslinking to improve the structural and functional properties of SBM, with significant implications for the agriculture and food industries.
Soybean meal is widely used in broiler diets due to its high amino acid content and digestibility. However, its practical applications are often limited by its low solubility, poor emulsification, and weak foaming properties. To address these challenges, Rouzban and her team employed a metagenomic laccase enzyme, PersiLac2, in combination with phenolic mediators caffeic acid (CA) and vanillic acid (VA), to crosslink wheat proteins—albumin (WAP) and globulin (WGP)—with soybean meal.
The results were striking. The crosslinking process significantly enhanced the water-holding capacity (WHC) of SBM from 4.34 to 5.25 g/g and the oil-holding capacity (OHC) from 0.74 to 1.12 g/g. Solubility also saw a marked improvement, rising from 33.6% to 47.6%. “The antioxidant activity of the crosslinked proteins increased dramatically, with ABTS and DPPH scavenging reaching 91% and 54%, respectively,” Rouzban noted. This enhancement in antioxidant properties is particularly noteworthy, as it opens up new avenues for developing functional foods with added health benefits.
The study also revealed substantial improvements in the foaming properties of the proteins. In wheat albumin (WAP), foaming capacity increased from 30% to 120%, and stability improved from 33% to 58%. For wheat globulin (WGP), vanillic acid (VA) raised foaming capacity to 44%, while the enzyme PersiLac2 improved stability to 33%. These enhancements are crucial for applications in the food industry, where foaming properties are essential for product texture and quality.
The research employed advanced analytical techniques such as SDS-PAGE, FTIR, and SEM to confirm the structural changes and successful crosslinking. SDS-PAGE revealed higher molecular weight aggregates, indicating effective crosslinking. FTIR and SEM analyses showed enhanced secondary structure stability and smoother surface morphology in the treated SBM, further validating the efficacy of the enzymatic modification.
The commercial implications of this research are profound. By improving the functional and nutritional quality of soybean meal, this method offers a sustainable biocatalytic route for upgrading protein quality in both food and feed industries. “This approach not only enhances the nutritional value of soybean meal but also provides a more sustainable and eco-friendly alternative to traditional chemical modifications,” Rouzban explained. The enhanced properties of SBM can lead to better feed formulations, improved animal health, and ultimately, higher productivity in the agriculture sector.
Looking ahead, this research paves the way for further exploration of enzymatic modifications in protein sources. The use of metagenomic laccases and natural phenolic mediators presents a promising avenue for developing innovative solutions to longstanding challenges in the food and agriculture industries. As the demand for sustainable and high-quality protein sources continues to grow, this study offers a glimpse into the future of protein modification and its potential to revolutionize the way we produce and consume food.
Published in *BMC Biotechnology*, this study was led by Tina Rouzban from the Department of Cell and Molecular Biology at Kharazmi University, highlighting the cutting-edge research being conducted in the field of agricultural biotechnology. The findings not only advance our understanding of protein modification but also offer practical solutions that can be implemented in commercial settings, shaping the future of the agriculture and food industries.