In the relentless battle against microbial threats, scientists are turning to an unexpected ally: plants. A recent study published in *Frontiers in Microbiology* has uncovered the multifaceted antimicrobial mechanisms of peptides derived from *Medicago truncatula*, a plant known for its symbiotic relationship with soil bacteria. The research, led by Hilda Tiricz from the Institute of Plant Biology at HUN-REN Biological Research Centre in Szeged, Hungary, could pave the way for innovative antimicrobial solutions in agriculture and beyond.
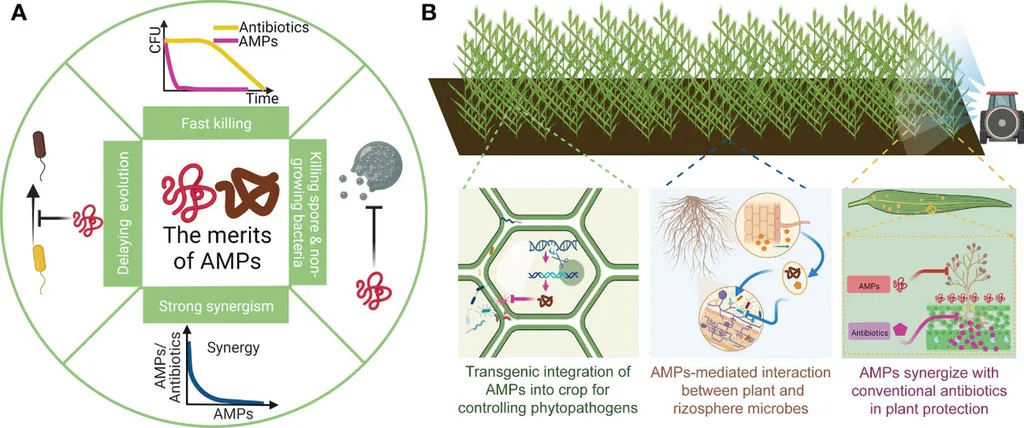
Antimicrobial peptides (AMPs) are a cornerstone of innate immunity, offering broad-spectrum activity against a variety of pathogens. In *Medicago truncatula*, over 700 nodule-specific cysteine-rich (NCR) peptides are produced, each with unique sequences and properties. While cationic NCRs are known for their potent antimicrobial activity, anionic NCRs typically lack this trait. NCR147, a neutral peptide, stands out as the only non-cationic NCR with weak bactericidal activity, making it a subject of intrigue for researchers.
Tiricz and her team synthesized 13 truncated and substituted derivatives of NCR147 to pinpoint the regions responsible for its antimicrobial activity. The results were promising. “The NCR147 derivatives displayed varying degrees of antimicrobial potency and spectrum,” Tiricz explained. “We found that the antimicrobial region resides in the C-terminal portion of these peptides, where a hydrophobic patch and positively charged amino acids contribute to their activity, likely through interactions with microbial membranes.”
The most active peptides not only altered bacterial membranes but also inhibited efflux pumps and interfered with essential intracellular targets. Notably, these peptides exhibited potent antibiofilm effects, preventing and degrading biofilms formed by *Acinetobacter baumannii*, a bacterium notorious for its resistance to antibiotics. The incorporation of 5-fluoro-L-tryptophan further enhanced the peptides’ antimicrobial breadth and antifungal activity, making them effective against pathogens like *Candida albicans* and *Cryptococcus neoformans*. Importantly, these modified peptides were non-cytotoxic to human cells, a critical factor for their potential therapeutic use.
The implications for the agriculture sector are significant. Biofilms, which are communities of microorganisms that adhere to surfaces, pose a substantial challenge in agricultural settings, leading to crop losses and reduced productivity. The ability of these NCR147-derived peptides to inhibit and eradicate biofilms could revolutionize crop protection strategies. “This research highlights the therapeutic promise of plant-derived AMPs as next-generation antimicrobials with reduced risk of resistance development,” Tiricz noted.
The study’s findings suggest that NCR147-derived peptides function through a multihit mechanism, targeting multiple pathways within microbial cells. This approach could mitigate the development of resistance, a growing concern in the era of antibiotic-resistant pathogens. As the world grapples with the challenges of sustainable agriculture and food security, the development of novel antimicrobials derived from plants offers a glimmer of hope.
The research, published in *Frontiers in Microbiology* and led by Hilda Tiricz from the Institute of Plant Biology at HUN-REN Biological Research Centre in Szeged, Hungary, opens new avenues for exploration in the field of antimicrobial therapies. By harnessing the power of plant-derived peptides, scientists are not only expanding our understanding of microbial defense mechanisms but also paving the way for innovative solutions to some of the most pressing challenges in agriculture and human health.