In the intricate dance of tumor progression and therapy resistance, a new player has taken center stage: the phosphatidylcholine ferroptosis axis. This emerging concept, detailed in a recent study published in *Frontiers in Molecular Biosciences*, sheds light on how lipid pathways and metabolic regulation orchestrate the crosstalk between cancer cells and their surrounding stroma. The research, led by Manikandan Vani Raju from the Department of Biochemistry at Karpagam Academy of Higher Education in Coimbatore, Tamil Nadu, India, offers a fresh perspective on how targeting these pathways could revolutionize cancer treatment and potentially impact the agriculture sector.
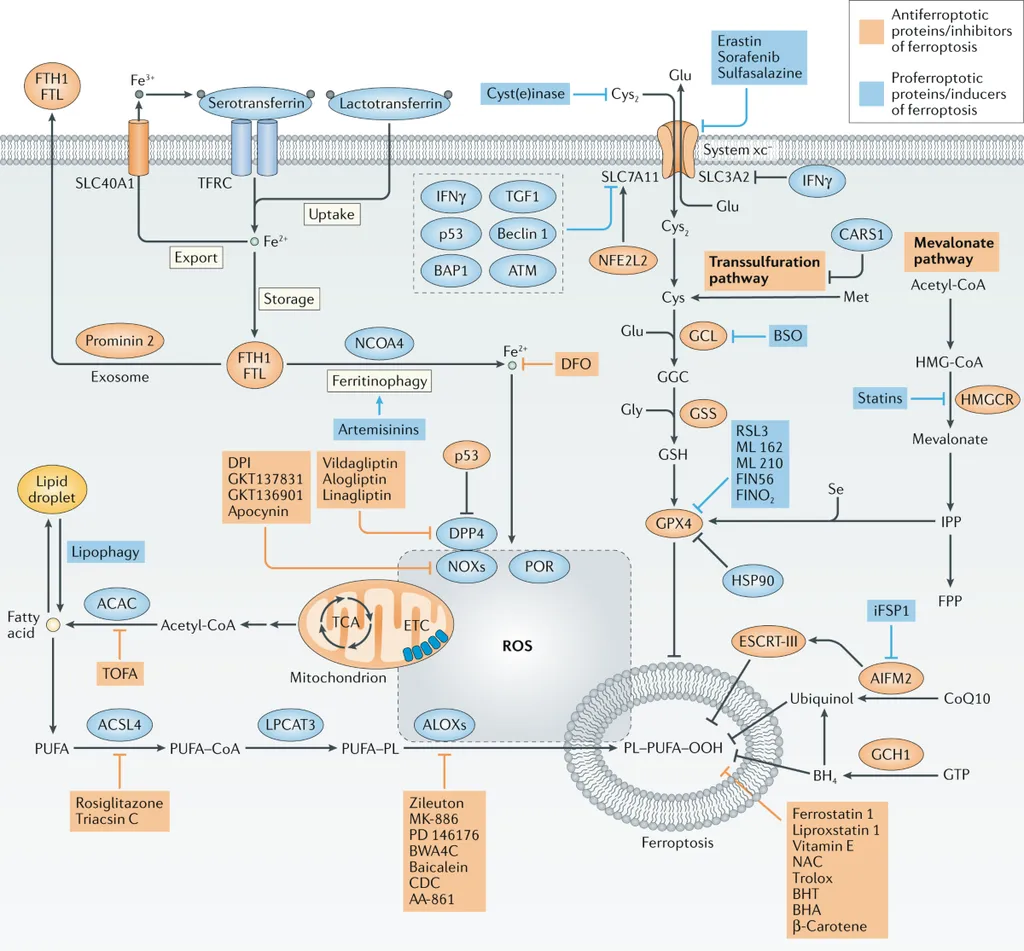
At the heart of this study is phosphatidylcholine, the most abundant phospholipid in mammalian membranes. “Enhanced phosphatidylcholine synthesis alters membrane architecture and generates substrates for lipid remodeling,” explains Raju. This remodeling process, driven by enzymes like lysophosphatidylcholine acyltransferases and phospholipase A2, influences the incorporation of polyunsaturated fatty acids (PUFAs) into membranes. These PUFAs are particularly prone to oxidative damage, a process known as ferroptosis—a regulated form of cell death driven by iron-dependent lipid peroxidation.
The implications of this research extend beyond the realm of cancer biology. In the agriculture sector, understanding lipid remodeling and ferroptosis could pave the way for innovative approaches to crop protection and enhancement. For instance, manipulating lipid pathways in plants could potentially enhance their resistance to oxidative stress, a common challenge in agriculture. This could lead to the development of more resilient crops, capable of withstanding environmental stressors and improving overall yield.
Moreover, the study highlights the role of the tumor microenvironment in regulating cancer cell fate. Cancer-associated fibroblasts and immune cells, such as macrophages and T cells, play crucial roles in modulating the phosphatidylcholine-ferroptosis axis. “Phosphatidylcholine metabolism extends beyond cancer cells to the tumor microenvironment,” notes Raju. This crosstalk between cancer cells and their stroma could offer new avenues for therapeutic intervention, potentially reprogramming stromal elements to enhance anti-tumor activity.
The commercial impacts of this research are profound. By targeting phosphatidylcholine synthesis and remodeling, combined with ferroptosis inducers or glutathione peroxidase 4 inhibitors, it may be possible to sensitize tumors to oxidative cell death. This approach could lead to the development of novel combination therapies designed to disrupt tumor-stroma crosstalk and improve clinical outcomes. In the agriculture sector, similar strategies could be employed to enhance crop resilience and productivity, addressing some of the pressing challenges faced by farmers worldwide.
As we delve deeper into the complexities of lipid metabolism and its role in cell fate, the phosphatidylcholine-ferroptosis axis emerges as a promising target for future therapies. The research led by Manikandan Vani Raju not only advances our understanding of cancer biology but also opens up new possibilities for innovation in the agriculture sector. By harnessing the power of lipid remodeling and ferroptosis, we may unlock new strategies to combat disease and enhance agricultural productivity, shaping a healthier and more sustainable future.